Chemistry for Biology
Acids and Bases
The pH scale is utilized to measure how acidic (acidity) and basic (alkalinity) a substance is. It is a logarithmic scale where each unit change in the pH scale corresponds to a ten-fold change in the [H+], or hydrogen ion concentration..

The pH of a solution can be calculated by the formula:
pH = -log10[H+]
or
pOH = -log10[OH-], and pH +pOH = 14
The pH Scale
where [H+] and [OH-] is measured in moles per liter of solution.
Acids and Bases
An acid is a proton (H+) ion donor that increases the concentration of H+ ions in a substance. A base is a proton acceptor or hydroxide donor that decreases the concentration of H+ ions in a substance and increases the concentration of OH- ions in a solution.
The pH of an aqueous solution ranges from 1 – 14, where a pH below 7 is called acidic, a pH above 7 is called basic, and a pH of 7 is called neutral.
In other words, an acidic solution has a concentration of [H+] > 10^-7 and [OH-] < 10^-7.
A basic solution has a concentration of [H+] < 10^-7 and [OH-] > 10^-7.
At equilibrium, [H+] = [OH-] = 10^-7. The more acidic a solution is, the greater the concentration of H+ ions. The less acidic a solution is, the smaller the concentration of H+ ions.
To calculate the pH of an aqueous solution:
Kw = [H+] x [OH-], where Kw = 10-14
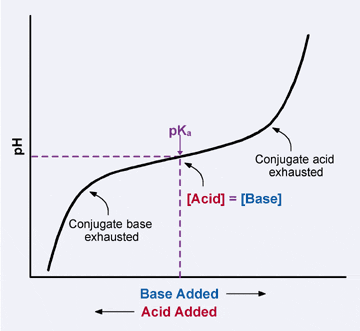
Buffers
A buffer is a solution that is resistant to pH change upon the addition of an acid or base. It is capable of neutralizing small amounts of acid or base, thus maintaining the pH of the solution. They are vital in processes that require a stable pH to function. Buffers each have a “buffer range,” where they can operate and neutralize acids and bases before the pH changes.
Buffers are generally weak acids or weak bases because they can donate or accept H+ ions as the pH decreases or increases.
Carbonic-acid-bicarbonate Buffer in Blood
This buffer is of importance in maintaining a blood pH of 7.4 in the human body.
Under normal standards, there is more bicarbonate that carbonic acid present in the blood (a ratio of 20:1). This is due to the body's metabolism, which generates more acids than bases to fulfill the body's needs. The blood is then able to neutralize metabolic acids due to its high concentration of OH- ions. Because fewer bases are produced, there is a smaller concentration of carbonic acid.