Chemistry for Biology
Ionic Bonds in crystal:
Ionic bonds are created by the transfer of valence electrons from a metal to a nonmetal, causing a large electronegativity difference between the atoms. The metal becomes a cation (donate electron and become positive) and the nonmetal becomes an anion (accepts electron and becomes negative). Ionic bonds have an electronegativity difference of 1.5 or greater.
In a non-aqueous solution, ionic bonds in crystals have high bond strength.
Ex: Sodium chloride (NaCl), Sodium fluoride (NaF)
Ionic Bonds in water:
The only difference with ionic bonds in water is that water often weakens ionic bonds
and causes them to break. This is a result of the weak bond strength of ionic bonds.
The bond strength of ionic bonds in water is about 3-7 kcal/mol. When in low concentration
in aqueous solutions, ionic bonds break easily, making them weaker than covalent bonds.
ex: NaCl in water
Hydrogen Bonds:
Hydrogen bonds are a form of dipole-dipole interactions, consisting of an inter- and intramolecular
electronegative attraction between Hydrogen and Fluorine, Oxygen, or Nitrogen.
Bond strength ranges from 3-7 kcal/mol.
ex: H bonds of DNA, RNA and proteins, H bonds in between water molecules
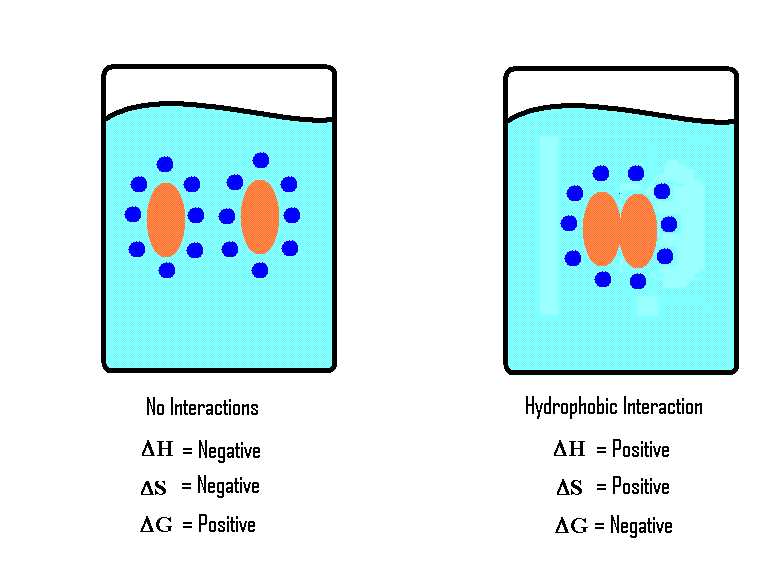
Hydrophobic interactions in aqueous solutions:
To reduce contact with water, non-polar, lipid-soluble hydrophobes clump together.
This results in attraction between hydrophobes in an aqueous solution.
Bond strength depends on temperature, number of carbon and shape of hydrophobes
ex: hydrophobic tail in membrane bilayer
Van der Waals interactions in aqueous solutions:
Also known as dispersion, london, temporary or induced dipoles.
Van der waal interactions are interactions between molecules with pockets of constantly changing positive and negative charges because of changing distributions of electron clouds.
Bond strength is dependent on molecular size and shape but is relatively, about 1 kcal/mol.
ex: cellulose in plant cell walls, lipids in biological membranes